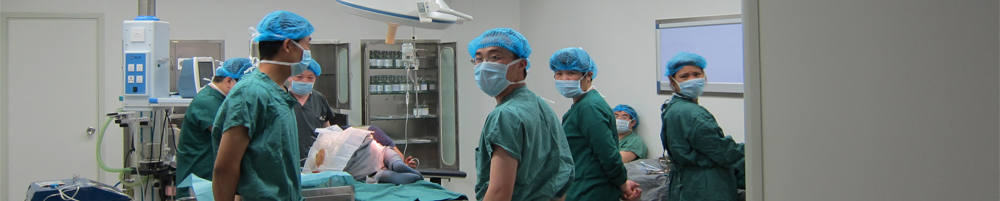
■ 中国药品生产洁净室(区)的空气洁净度标准
|
洁净度级别 |
尘埃最允许数/平方米 |
微生物最大允许数 |
| |
≥0.5um |
≥5um |
浮游菌个/立方米 |
沉降菌个/皿.30min |
|
100 |
3500 |
0 |
5 |
1 |
|
10000 |
350,000 |
2,000 |
100 |
3 |
|
100000 |
3,500,000 |
20,000 |
500 |
10 |
|
300000 |
10,500,000 |
61,800 |
NA |
15 | |
■ 基于≥0.5um粒径的各国洁净度等级近似对照表
|
个/M3≥0.5um |
ISO
14644-1(1999) |
US
209E(1992) |
US
209D(1988) |
EEC
GGMP(1989) |
FRANCE
AFNOR(1981) |
GERMANY
VDI 2083(1990) |
JAPAN
JAOA(1989) |
|
1 |
- |
- |
- |
- |
- |
- |
- |
|
3.5 |
2 |
- |
- |
- |
- |
0 |
2 |
|
10.0 |
- |
M1 |
- |
- |
- |
- |
- |
|
35.3 |
3 |
M1.5 |
1 |
- |
- |
1 |
3 |
|
100 |
- |
M2 |
- |
- |
- |
- |
- |
|
353 |
4 |
M2.5 |
10 |
- |
- |
2 |
4 |
|
1,000 |
- |
M3 |
- |
- |
- |
- |
- |
|
3,530 |
5 |
M3.5 |
100 |
A+B |
4,000 |
3 |
5 |
|
10,000 |
- |
M4 |
- |
- |
- |
- |
- |
|
35,300 |
6 |
M4.5 |
1,000 |
1,000 |
- |
4 |
6 |
|
100,000 |
- |
M5 |
- |
- |
- |
- |
- |
|
353,000 |
7 |
M5.5 |
10,000 |
C |
400,000 |
5 |
7 |
|
1,000,000 |
- |
M6 |
- |
- |
- |
- |
- |
|
3,530,000 |
8 |
M6.5 |
100,000 |
D |
4,000,000 |
6 |
8 |
|
10,000,000 |
- |
M7 |
- |
- |
- |
- |
- | |